Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
Making lithium-ion batteries more environmentally friendly
New process uses water-soluble binders to avoid the need for organic solvents in manufacturing and recycling
by Mitch Jacoby
April 30, 2020
| A version of this story appeared in
Volume 98, Issue 17
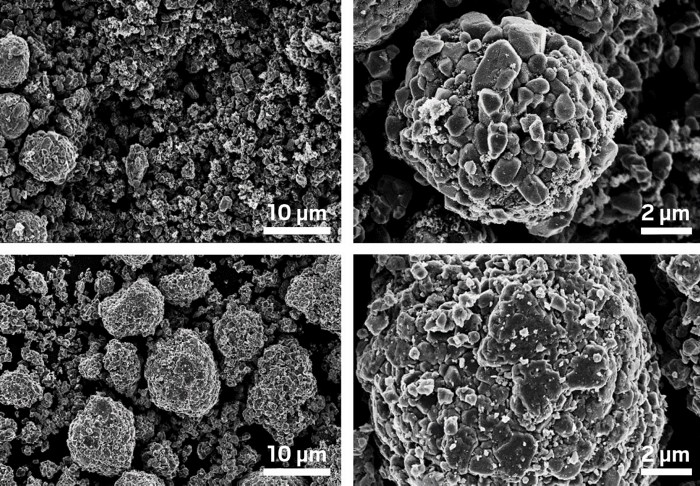
By reformulating the materials used for manufacturing lithium-ion batteries, researchers have come up with a way to process and recycle the batteries’ electrodes without using organic solvents (iScience 2020, DOI: 10.1016/j.isci.2020.101081). The advance could reduce the costs, hazards, and environmental impact of manufacturing and recycling these common power sources.
The soaring popularity of electric vehicles is generating enormous numbers of spent lithium-ion batteries and pushing manufacturers to make new ones. Industry analysts predict that more than 10 million metric tons of these batteries will reach the end of their service life by 2030. Due to technical and economic barriers, less than 5% of them are recycled today.
One key recycling hurdle is recovering and reusing the valuable metals found in the electrodes. Cathodes are typically made by gluing an electrochemically active mixed-metal powder to an aluminum current collector with a binder such as poly(vinylidene fluoride) (PVDF). Anodes usually contain graphite, PVDF, and copper foil. To use this and other standard binders, industry relies on N-methyl-2-pyrolidone (NMP)—a costly, toxic solvent—to process electrodes. NMP is also used to separate and recover the spent materials.
Researchers have tried for years, with limited success, to eliminate NMP, aiming instead for water-based processing. In the new study, a team led by Jianlin Li of Oak Ridge National Laboratory and Zheng Li of Virginia Tech replaced PVDF with a water-dispersible latex-based binder in the cathode and water-soluble styrene-butadiene in the anode, enabling them to use water-based-processing to make and recycle batteries. Through tests spanning thousands of charging cycles, the team showed that batteries made with water processing worked roughly as well as reference cells processed with NMP. Batteries recycled using water also worked nearly as well as pristine ones.
Ramin Amin-Sanayei, a polymer and battery specialist at Arkema, says industry is skeptical about water processing because unwanted reactions between lithium compounds and water can ruin battery performance. This study, however, “moves in the right direction and in a big way,” he says.
Experts agree that additional testing done under more demanding conditions will be needed to persuade battery makers to remove organic solvents from their processes. Argonne National Laboratory’s Linda L. Gaines, a specialist in materials and life-cycle analysis, says that’s a critical goal. “Avoiding the use of toxic chemicals is an important way to reduce the costs and environmental impacts of the lithium-ion battery life cycle.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter