New catalyst may hasten commercialization of fuel cell vehicles
If commercially viable, the new catalyst could replace platinum in electric cars powered by fuel cells instead of batteries, which would greatly extend the range of electric vehicles and eliminate the need for recharging.
Fuel cells generate electricity by using hydrogen from a fuel tank with oxygen in the air. The only waste product emitted to the environment is water.
But fuel cells are expensive, largely because they depend on the precious metal platinum to cause the hydrogen-oxygen reaction. Argonne’s fuel cell catalyst replaces much of the platinum with a non-precious metal.
“Platinum represents about 50 percent of the cost of a fuel cell stack, so replacing or reducing platinum is essential to lowering the price of fuel cell vehicles,” said Di-Jia Liu, who led the Argonne team. Their catalyst replaces all the platinum in the fuel cell’s cathode, which usually requires four times as much platinum as the anode, and their new electrode design also optimizes the flow of protons and electrons within the fuel cell and the removal of water.
Many automakers see sales of vehicles powered by fuel cells as eventually outpacing battery-powered electric vehicles for several reasons: fuel-cell vehicles emit only water, can travel over 300 miles between fill ups, can be refilled quickly and place no burden on the electrical grid because they don’t need recharging.
Since both technologies lack refilling or recharging infrastructures and are expensive, both are currently suitable mainly for early adopters and use in corporate fleets. But this may change, if advances made by Argonne researchers can be realized in commercial fuel-cell vehicles.
Fuel cells generate electricity to propel vehicles through electrochemical reactions between onboard hydrogen fuel and oxygen in the air. Hydrogen molecules are stripped of electrons at the fuel cell’s anode, becoming protons that travel through a polymer electrolyte membrane to the cathode, where they react with electrons and oxygen to form water.
“In order for a fuel cell to work,” Liu explained, “the catalyst must be densely packed with active sites that are uniformly distributed throughout the cathode and directly connected to the arriving protons and electrons, while maintaining easy access to oxygen. The catalyst should also have an architecture that can readily channel away the produced water.” No conventional method for preparing carbon-based platinum or non-precious metal catalysts can meet all these criteria, Liu added.
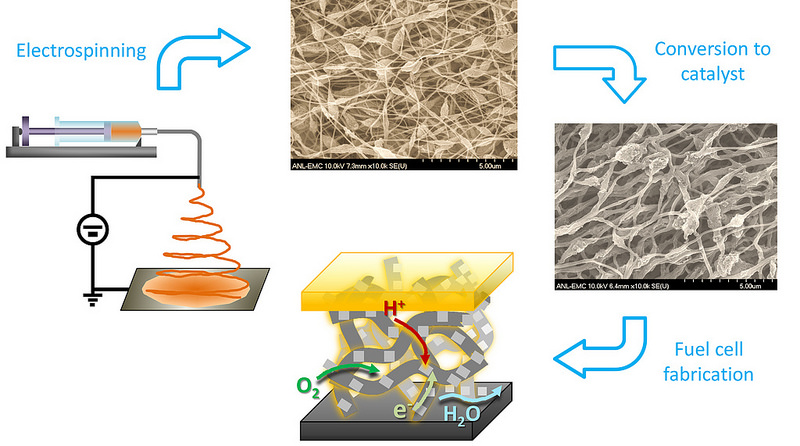
In a paper recently published in the Proceedings of the National Academy of Sciences of the United States of America, the team led by Liu reported on a new method of synthesizing a highly efficient, nanofibrous non-precious metal catalyst by electrospinning a polymer solution containing a mixture of ferrous organometallics and metal-organic frameworks. Following thermal activation, the new catalyst delivered an unprecedented level of catalytic activity in actual fuel cell tests.
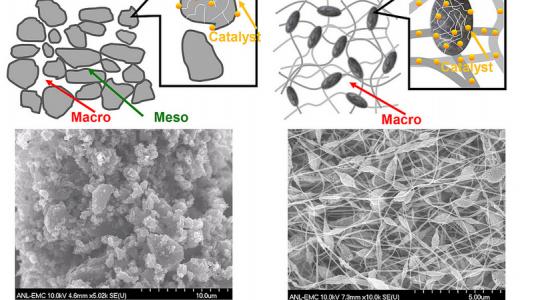
“The new catalyst offers a unique carbon nano-network architecture made of microporous nanofibers interconnected through a macroporous framework,” Liu explained. “Not only do the active sites inside the micropores within individual fibers catalyze chemical reactions effectively, but the macroporous voids between the fibers transport oxygen and water efficiently to and from the active sites. The continuous nano-networks also make the catalytic electrode highly conductive in charge transfer.”
The paper, “Highly efficient nonprecious metal catalyst prepared with metal–organic framework in a continuous carbon nanofibrous network,” was published online on August 10, 2015.
The research was supported by the U.S. Department of Energy’s Office of Science and the Office of Energy Efficiency and Renewable Energy, Fuel Cell Technologies Office.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. With employees from more than 60 nations, Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. Argonne is supported by the Office of Science of the U.S. Department of Energy.
The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.