APS Upgrade to enhance ‘molecular movies’ to understand certain types of antibiotic resistance
New technique deployed to visualize biological reactions in real time
Eadweard Muybridge’s electrifying photos of a galloping horse set the world on fire when he created the precursor to what became motion pictures. For today’s scientists, a new upgrade to one of the world’s most powerful hard X-ray light sources could improve the way molecular movies are made. These could reveal hidden secrets of different chemicals, potentially paving the way for new treatments and pharmaceuticals.
Scientists typically use different forms of a technique called crystallography to reconstruct the molecular structure of proteins. Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have used and expanded a new method called serial crystallography, developed previously at X-ray free-electron laser facilities.
“With the APS Upgrade, we will have the ability to study all sorts of reactions as they happen, especially for biological systems.” — Andrzej Joachimiak, Argonne National Laboratory and University of Chicago
Combining serial crystallography with observations over short time scales (from a tenth to a hundredth of a second) allows scientists to detect real-time changes in the shape of proteins and bound molecules during chemical reactions. The technique offers a unique advantage over previous forms of crystallography, as individual crystals can be smaller and they only need to be illuminated with X-ray beams once, and for a short period of time.
The upcoming upgrade to Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility at Argonne, will create X-ray beams that are up to 500 times brighter than those currently generated at the facility. This will allow for serial crystallography to be more broadly available at the APS, said Argonne distinguished fellow Andrzej Joachimiak, who is also the director of the Structural Biology Center (SBC) at the APS and a professor at the University of Chicago.
“Serial crystallography is really in its infancy and has largely been the purview of specialized facilities with free-electron lasers,” said Joachimiak. “With the APS Upgrade, we will have the ability to study all sorts of reactions as they happen, especially for biological systems.”
In a recent experiment in conjunction with researchers at the University of Chicago, Joachimiak used serial crystallography to examine the reaction of an antibiotic drug and an enzyme isolated from a drug-resistant pathogen. The result could help give researchers a better idea of the molecular mechanisms that enable certain bacteria to gain antibiotic resistance.
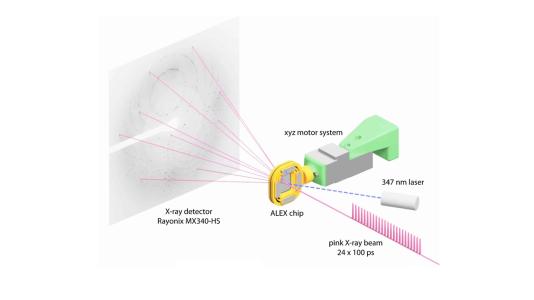
The research team made use of SBC at beamline 19-ID and the specialized capabilities of the BioCARS beamline at 14-ID, managed by the University of Chicago. According to Vukica Srajer, a BioCARS research beamline scientist and a co-author on the paper, the beamline is one of a few in the world that can perform serial crystallography on incredibly short time scales. Researchers used BioCARS to perform time-resolved serial crystallography.
By doing an X-ray scan of a plastic chip that contained a suspension of thousands of individual protein microcrystals, Joachimiak and his colleagues were able to quickly and accurately reconstruct several protein structures. The researchers used a “pump-probe” technique in which they shined ultraviolet light on the sample to initiate a reaction. They then used the X-ray beam to observe the result at different time points.
“Previous attempts at doing this kind of crystallography would destroy the crystals before we could get the complete data,” Joachimiak said. “Because we’re only shining the X-ray beams on each particular crystal for a very short period of time, we can get over 40,000 images from one chip. This dramatically speeds up our crystallography efforts and gives us the ability to see into protein mechanisms over several time scales we’d never before been able to resolve.”
The advantage of doing serial crystallography, according to Joachimiak, is that it allows scientists to observe changes in the protein’s structure as they happen. In the case of an enzyme, it also gives scientists the ability to look at how the enzyme’s active site interacts with another molecule, or substrate.
In the study, Joachimiak and his colleagues looked at a protein-enzyme complex called a beta-lactamase, which gives certain pathogens antibiotic resistance. With serial crystallography, the researchers were able to notice a buildup of zinc atoms that triggered the enzyme to break through the antibiotic molecule.
“It’s as if you were trying to open a jar with a stuck lid,” Joachimiak said. “You keep twisting and twisting and it doesn’t move initially, until ultimately it suddenly gives way.”
According to Joachimiak, a water molecule becomes activated by zinc ions to break the bond of the antibiotic. “Serial crystallography shows us exactly when the zinc makes the reaction happen,” he said. “You can watch it happen in real time.”
Mateusz Wilamowski, a researcher at Jagellonian University in Poland and a former postdoctoral researcher at the University of Chicago, who also helped perform the research, said that the ability to resolve the dynamics of this particular class of molecules could have broad-reaching implications. “There are many other proteins like this that rely on similar mechanisms,” he said. “Nobody has been able to study the intermediate transitions of the molecule that we were able to visualize.”
Regular protein crystallography does not allow for the creation of these molecular movies because scientists can only collect a handful of images before destroying the crystal, Joachimiak explained. “It’s truly a revolutionary technique that will have a huge impact on how we can observe and ultimately design better drugs,” he said.
Wilamowski also believes that the results will help enable intelligent drug design, as future research could pair the APS Upgrade with quantum mechanical calculations to improve already existing molecules.
A paper based on the study appeared Nature Communications.
The work was funded by DOE’s Office of Science (Office of Biological and Environmental Research) and the National Institutes of Health.
About the Advanced Photon Source
The U. S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. The APS provides high-brightness X-ray beams to a diverse community of researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. These X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being. Each year, more than 5,000 researchers use the APS to produce over 2,000 publications detailing impactful discoveries, and solve more vital biological protein structures than users of any other X-ray light source research facility. APS scientists and engineers innovate technology that is at the heart of advancing accelerator and light-source operations. This includes the insertion devices that produce extreme-brightness X-rays prized by researchers, lenses that focus the X-rays down to a few nanometers, instrumentation that maximizes the way the X-rays interact with samples being studied, and software that gathers and manages the massive quantity of data resulting from discovery research at the APS.
This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology by conducting leading-edge basic and applied research in virtually every scientific discipline. Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.