Scientists discover a potential ‘diamond factory’ near the center of the Earth

When steel reacts with water at the Earth’s surface, it rusts. But what happens deep inside the Earth’s interior?
Scientists working at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility, sought to find out. Located at DOE’s Argonne National Laboratory, the APS provides researchers with the opportunity to conduct experiments at the extremely high temperatures and pressures that exist near Earth’s core.
“To simulate the extreme conditions at Earth’s core-mantle boundary, we squeezed a tiny amount of materials between two diamond anvils to reach pressures of millions of atmospheres and heated the samples to thousands of degrees with powerful lasers” — Vitali Prakapenka, University of Chicago
The Earth’s core is the largest carbon storage on Earth — it’s suggested that roughly 90% of the world’s carbon is buried there. Scientists have shown that the oceanic crust that sits on top of tectonic plates and moves into the interior (in a process called subduction) contains hydrous minerals. These minerals, which contain water in their chemical structure, can sometimes descend all the way to the core-mantle boundary.
The temperature at the core-mantle boundary is at least twice as hot as lava, hot enough that water can be released from the hydrous minerals. Therefore, a chemical reaction similar to rusting steel could occur when water hits the molten metal at Earth’s core-mantle boundary.
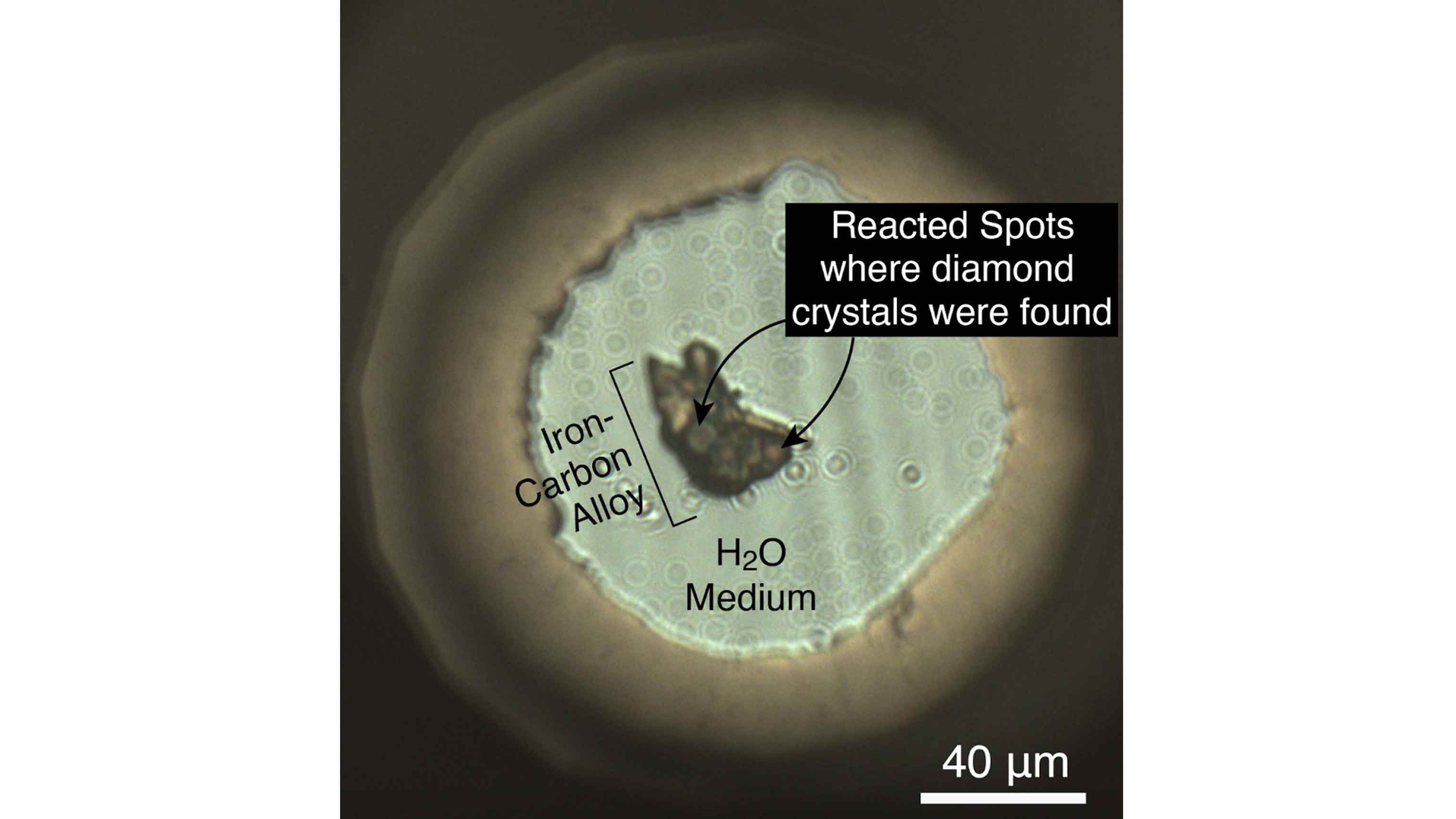
Postdoctoral researcher Byeongkwan Ko and his colleagues at Arizona State University’s School of Earth and Space Exploration came to the APS to discover what these reactions might look like deep within the Earth. They worked with colleagues at the GeoSoilEnviroCARS (GSECARS) beamline, which is operated by The University of Chicago. At the beamline, they compressed iron-carbon alloy and water (ice at high pressure) together to the pressure and temperature expected for Earth’s core-mantle boundary, melting the alloy.
The team found that water and metal react and make iron oxides and iron hydroxides, just like rusting at Earth’s surface. However, they found that at the conditions of the core-mantle boundary, unlike rusting at Earth’s surface, carbon comes out of the liquid iron metal alloy and forms diamond.
Their findings were recently published in the journal Geophysical Research Letters.
“To simulate the extreme conditions at Earth’s core-mantle boundary, we squeezed a tiny amount of materials between two diamond anvils to reach pressures of millions of atmospheres and heated the samples to thousands of degrees with powerful lasers. This induced chemical reactions and phase transitions not possible at ambient conditions,” said UChicago’s Vitali Prakapenka, a GSECARS beamline scientist and a co-author on the paper.
Analyzing the products of those reactions is no easy task, he said. “We have to use a combination of a superbright and tightly focused X-ray beam, laser techniques and high-resolution spectroscopy tools. It was that unique set of techniques and equipment available at our beamline at APS that allowed us to detect the formation of the diamond phase under the core-mantle conditions.”
Because iron has a high affinity for carbon, significant carbon is expected to exist in the core, while the mantle is thought to have relatively low carbon. However, scientists have found that much more carbon exists in the mantle than expected.
“Carbon is an essential element for life and plays an important role in many geological processes. The new discovery of a carbon transfer mechanism from the core to the mantle will shed light on the understanding of the carbon cycle in the Earth’s deep interior,” Ko said. “This is even more exciting given that the diamond formation at the core-mantle boundary might have been going on for billions of years.”
Ko and his team will continue their work at APS. They plan on investigating how the reaction can also change the concentration of the other light elements in the core, such as silicon, sulfur and oxygen, and how such changes can impact the mineralogy of the deep mantle.
A version of this release was originally posted by Arizona State University.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology by conducting leading-edge basic and applied research in virtually every scientific discipline. Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.