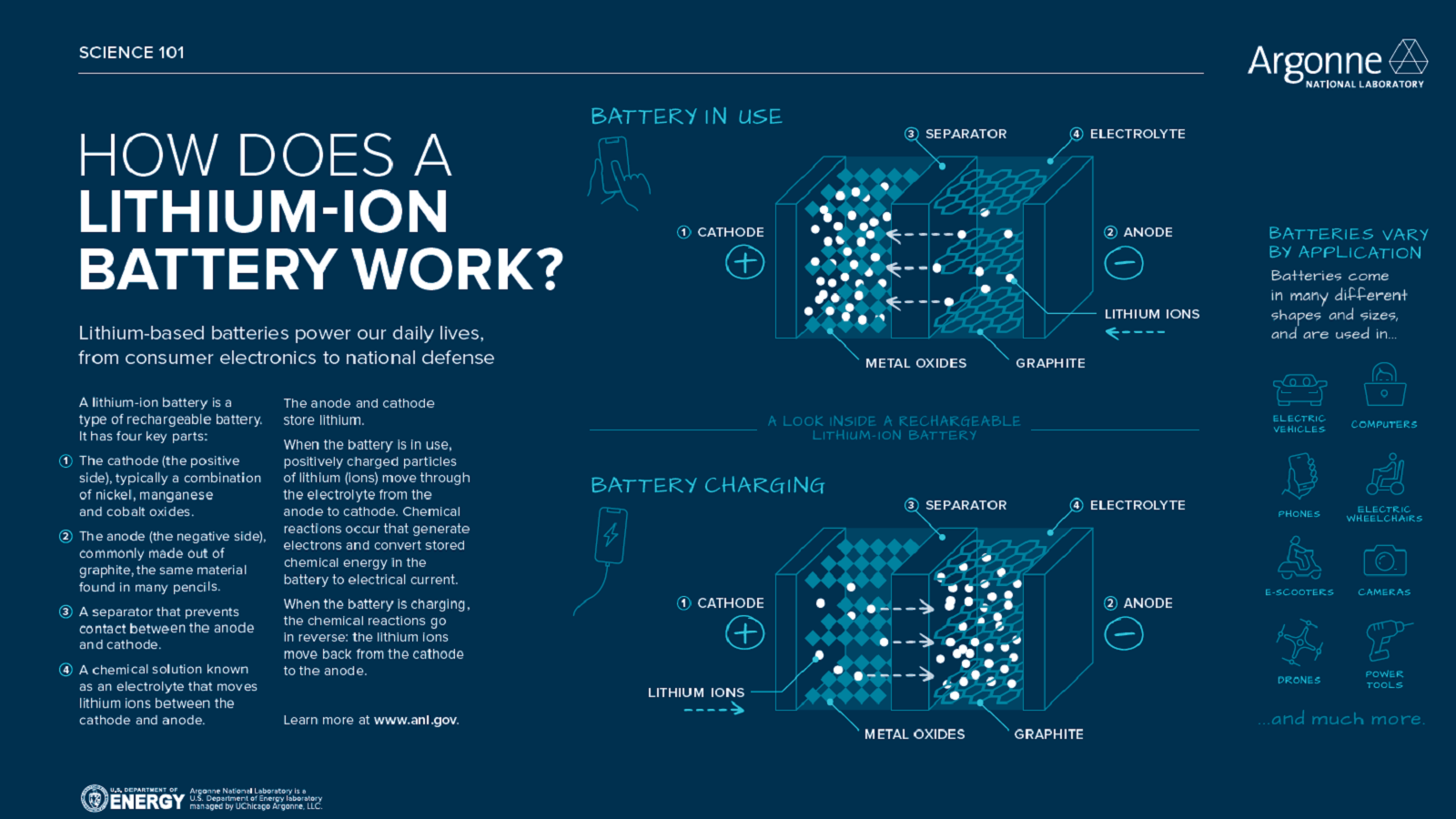
Exploring next-generation battery technologies: Q&A with Eungje Lee

The quest for batteries that are safer and made from earth-abundant resources has taken center stage. For decades, lithium-ion batteries have powered our lives, fueling everything from smartphones to electric vehicles. However, their reliance on flammable liquid electrolytes poses safety concerns and limits their applications in critical sectors. As a result, scientists and researchers are exploring alternative options that could eventually replace these ubiquitous power sources.
Eungje Lee, materials scientist at Argonne National Laboratory, has two contenders on his research radar: solid-state batteries and sodium-ion batteries. Solid-state batteries use a thin, solid film to separate their charge-generating components, eliminating the need for flammable liquid electrolytes.
In contrast, sodium-ion batteries present a potentially abundant and sustainable alternative. Sodium, a more readily available resource than lithium, could alleviate concerns over resource scarcity and pave the way for a greener future.
In the Q&A below, Lee discusses the promise of solid-state and sodium-ion batteries, along with challenges in their commercialization and widespread adoption.
Q: Current lithium-ion batteries have made significant progress. Can you explain why we need to explore alternative battery technologies?
A: Over the past decades, lithium-ion batteries based on transition metal oxide cathodes, graphite anodes, and organic liquid electrolytes have shown remarkable progress in increasing their energy density. However, the traditional approach of making incremental improvements to existing materials and processes is reaching its practical limitation. And despite the potential of next-generation high-energy electrode materials, such as lithium-rich cathodes and silicon anodes, we still face fundamental challenges of safety and cost.
Safety is a major concern when attempting to pack more and more high-energy materials into a limited battery volume. The growing number of battery fire incidents highlights the need for safer solutions. Additionally, the cost of lithium-ion batteries is significantly influenced by the cost of key materials like lithium, cobalt, and nickel. Fluctuations in their supply chain pose a threat to the reliable and cost-effective manufacturing of batteries. Considering these, it’s evident that merely making incremental improvements to existing materials and processes will not address the underlying challenges. Instead, we need to explore alternative battery chemistries and architectures that can offer different approaches and capabilities.
One such alternative is solid-state batteries, which use solid electrolytes instead of flammable liquid electrolytes, enhancing safety and potentially increasing energy density. Sodium-ion batteries are another promising option that can provide a more abundant and cost-effective alternative to lithium-ion batteries.
Q: Solid-state batteries have gained significant attention for their potential safety and energy density improvements. What are the major scientific and engineering hurdles in realizing their full potential?
A: Solid-state batteries have garnered significant attention due to their potential for improved safety and energy density. However, there are several scientific and engineering hurdles that need to be overcome to realize their full potential.
One major challenge lies in finding solid electrolytes that exhibit both high ionic conductivity and excellent chemical and electrochemical stability. The ideal solid electrolyte should be stable when in contact with high-voltage cathodes and low-voltage lithium anodes. Identifying materials that possess these desired properties remains a significant scientific challenge.
Another hurdle is related to materials production. It’s essential to determine which solid electrolyte materials can be produced in bulk and establish efficient manufacturing methods. For example, producing the precursor material lithium sulfide, commonly used in the synthesis of sulfide-based electrolytes like lithium phosphorus sulfide, can be challenging despite the earth-abundant nature of the electrolyte itself. Similarly, electrolytes like lithium lanthanum zirconium oxide require the rare-earth metal lanthanum, which may pose difficulties in obtaining and producing the necessary precursors at the scale required for gigawatt-hour (GWh) production.
Device manufacturing presents another significant challenge. It may be necessary to redesign or deviate from standard battery formats for electrodes and full cells in solid-state battery production. Material-specific challenges will arise, but there are shared manufacturing questions that cut across different material systems. This includes considerations such as the rheology and chemical compatibility of slurries for solid electrolytes or catholytes (solid electrolyte-containing cathodes). Implementing a dry processing route is another avenue of investigation. Additionally, there are significant questions surrounding the manufacturing of thin lithium metal that meets the battery’s requirements without excessive cost.
At the cell and pack level, maintaining mechanical integrity throughout the manufacturing and operation of solid-state batteries is a key challenge. Unlike liquid-based batteries, solid-state batteries lack the ability to relieve stress accumulation caused by volume expansion and contraction during battery operation. Therefore, it becomes essential to engineer mechanisms that can address this issue and ensure the mechanical stability of solid-state batteries.
Addressing these scientific and engineering challenges in solid-state battery technology is crucial to fully realize their potential for enhanced safety and energy density. Continued research and development efforts at Argonne are focused on finding suitable solid electrolytes and zero-strain cathodes, developing functional interfaces, and improving manufacturing processes to overcome these hurdles and enable the widespread adoption of solid-state batteries.
Q: What advantages do sodium-ion batteries offer over traditional lithium-ion batteries, and what are the main obstacles in their commercialization and widespread adoption?
A: One of the key advantages of sodium-ion batteries is the abundance and wide geographical availability of sodium resources. Compared to lithium, sodium is more abundantly available in nature and can be sourced from various deposits and brine sources. This abundance of sodium resources can potentially reduce the cost and dependency on limited lithium reserves.
Furthermore, sodium chemistry allows for the use of other abundant elements in cell components. For example, being larger than lithium in size, sodium doesn’t easily mix with transition metal elements and enables the use of earth-abundant iron and manganese in oxide cathodes instead of expensive cobalt and nickel. And unlike lithium, sodium does not alloy with aluminum, allowing for the use of less expensive aluminum as both the cathode and anode current collector in sodium-ion batteries. The use of corrosion-resistive aluminum current collector in anode side enables safer storage and transportation by completely de-energizing the cell through zero-voltage discharge.
Another advantage of sodium-ion batteries is the similarities in cell chemistry with lithium-ion batteries, which allows existing infrastructure and manufacturing processes for lithium-ion batteries to be adapted for sodium-ion battery production. This could facilitate the commercialization of sodium-ion batteries by leveraging the established battery industry.
“We need to explore alternative battery chemistries and architectures that can offer different approaches and capabilities.” — Eungje Lee, Argonne materials scientist
However, there are obstacles that need to be addressed for the commercialization and widespread adoption of sodium-ion batteries. Sodium-ion batteries currently have lower energy density compared to lithium-ion batteries, which limits their application in certain high-energy-demanding devices. Improving the energy density of sodium-ion batteries while maintaining their other advantages, such as cost-competitiveness, is a significant challenge.
Anodes for sodium-ion batteries also pose a significant challenge as the most developed anode materials in lithium-ion batteries, such as graphite and silicon, do not store sodium ions. Although alternative anode materials like hard carbon have been developed, their performance still needs further improvement to achieve higher capacity and energy density.
Cycle life and durability are other challenges faced by sodium-ion batteries. The materials used in sodium-ion batteries can experience larger structural changes during cycling, leading to capacity degradation. Additionally, the lack of high-quality sodium electrolyte materials slows down the development of high-performance sodium-ion batteries. The impact of electrolyte chemistry and purity on the electrode-electrolyte interface needs further investigation.
Furthermore, sodium-containing materials are more sensitive to ambient air exposure, requiring stricter control of materials handling and production environment, which can increase manufacturing costs. Improving storage and calendar-aging stability through compositional modifications and surface coating technologies is an important area of exploration.
To overcome these obstacles, continuous research and development efforts, advancements in materials science, improvements in cell design, and collaboration between academia, industry, and government entities are crucial. With further advancements, sodium-ion batteries hold promise as an alternative energy storage technology in the future.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology by conducting leading-edge basic and applied research in virtually every scientific discipline. Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.