Argonne collaborates with Toyota on innovative battery recycling process
The U.S. Department of Energy’s (DOE) Argonne National Laboratory and Toyota Motor North America — including its Michigan-based Toyota Research Institute of North America (TRINA) — are advancing an innovative “direct” recycling approach for electric vehicle (EV) batteries.
In most battery recycling today, the chemical structure of end-of-life battery components is broken down into the raw materials used in manufacturing. A new technique called direct recycling carefully extracts the components from spent batteries, retaining the components’ original structure. Manufacturers can re-use the components, reducing costs, waste and consumption of pristine materials.
The Argonne-Toyota collaboration is taking advantage of an effective, patent-pending direct recycling process developed by the Argonne-based ReCell Center, which is funded by the DOE’s Vehicle Technologies Office. The process uses a magnet to separate a battery’s high-value cathodes (positive electrodes) from other components.
Toyota is providing Argonne with Toyota plug-in hybrid EV batteries. Argonne will test the process on the batteries, and both Argonne and Toyota will measure the performance of the resulting cathodes. The goal is to prove that the process can make high-performing cathodes cost-effectively while reducing energy use and emissions. If the project is successful, EV and battery manufacturers can potentially commercialize the process, helping to make domestic battery supply chains more robust and circular.
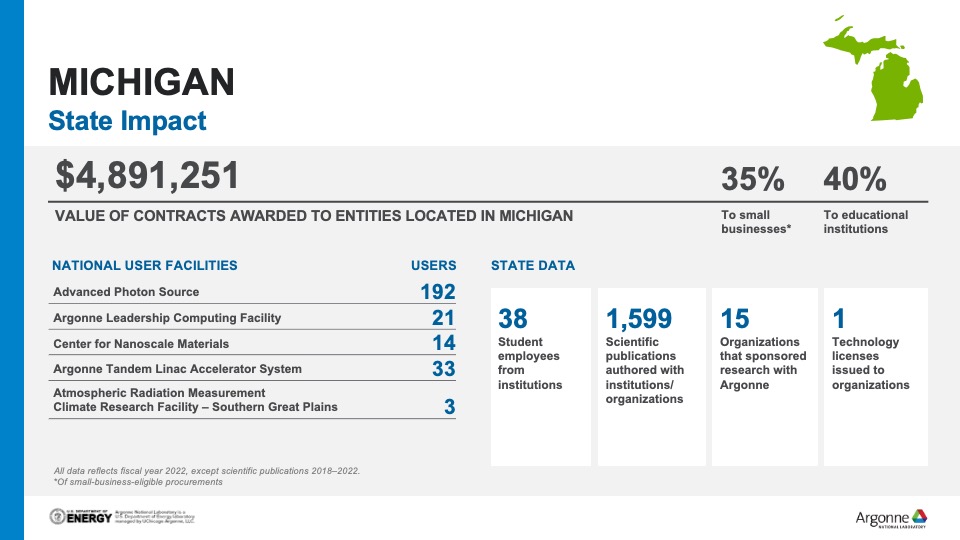
Michigan and Argonne join forces to drive clean energy transition
Using cutting-edge scientific research to realize a cleaner and more resilient Michigan economy is the aim of a newly formed partnership between the state and Argonne.
The partnership focuses on researching, developing, demonstrating and deploying technologies in four areas: industrial decarbonization, battery manufacturing and recycling, workforce development and future mobility systems planning. Investment decisions involving the decarbonization of Michigan’s industrial base, which includes manufacturing products such as iron and steel, cement, chemicals, forest products and furniture, will be informed by Argonne’s expertise in energy efficiency, industrial electrification, low-carbon fuels, and carbon capture and storage.
“As a national laboratory, Argonne is uniquely positioned to provide research-backed insights to support Michigan’s efforts,” said Claus Daniel, associate laboratory director for Advanced Energy Technologies at Argonne. “We look forward to applying our expertise to support the state as it seeks to grow investment in key sectors and prepares a diverse talent pipeline for emerging sectors.”
Argonne’s energy storage research — focused on reducing dependence on critical materials, facilitating sustainable resource recovery, discovering new lower-cost and higher energy density materials and enabling safe fast charging — will inform decisions as Michigan advances economic expansion in battery manufacturing and assembly.
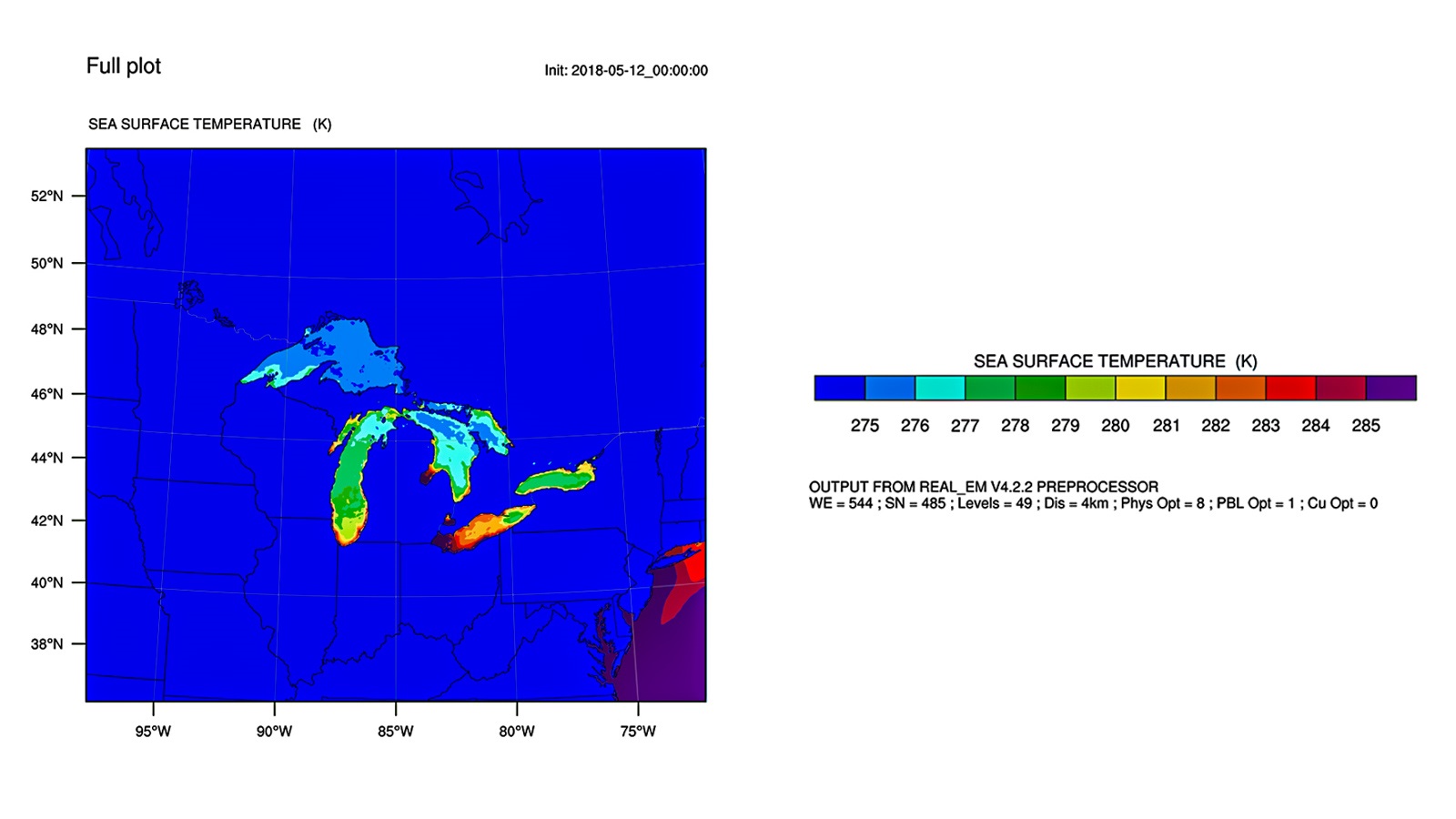
Improved weather and climate models predict the future of the Great Lakes region
Jiali Wang from DOE’s Argonne is part of a project called Coastal Observations, Mechanisms, and Predictions Across Systems and Scales (COMPASS), funded by the Office of Biological and Environmental Research in the U.S. Department of Energy’s Office of Science.
In a new study, Wang and a team of collaborators designed high resolution regional model experiments to explore how lake surface temperatures may affect the climate of the Great Lakes region.
The study indicates that rising lake surface temperatures have the potential to destabilize regional climate conditions throughout the Great Lakes basin. This could increase extreme weather events, causing larger storms and flooding in an area that is home to 30 million people.
Accurate regional and global climate models, as informed by the work of COMPASS, will be crucial for understanding how climate change will affect our lives and infrastructure in the next twenty to thirty years. They can also help shape the country’s plans for climate resilience.
Michigan Tech, Argonne partner on a pivotal discovery: economical battery recycling
Researchers at Michigan Technological University in Houghton are partnering with a team including scientists at Argonne to make it more economical to recycle a lithium-ion battery. Scientists at Argonne’s Materials Engineering Research Facility are scaling up Michigan Tech’s pivotal discovery — an innovative process for separating and recovering the valuable cathode materials in a battery — paving the way for economical recycling.
Their work is conducted at the ReCell Center, the nation’s first advanced battery research and development center, funded by DOE’s Vehicle Technologies Office and housed at Argonne. The center is a collaboration that, in addition to Michigan Tech, includes the DOE’s National Renewable Energy Laboratory and Oak Ridge National Laboratory, the University of California at San Diego and Worcester Polytechnic Institute, Worcester, Massachusetts.
In the journal Energy Technology, researchers described how they separated individual cathode materials in a new twist on an old process called froth flotation. Long used in the mining industry to purify ores, froth flotation separates materials in a flotation tank based on whether a material repels water; sticks to air bubbles and floats; or attracts water, is not affected by air bubbles and sinks.
The discovery promises wide-ranging implications for the lithium-ion battery industry, including cost reduction and a profitable recycling market, enabling U.S. competition in the global battery recycling industry, strengthening the nation’s energy independence and reducing dependence on foreign sources of materials.
Argonne physicist, MSU partner to explore how elements are formed
Physicist Benjamin Kay at Argonne is leading a team of scientists at Michigan State University (MSU) in East Lansing, using a device that promises to reveal new insights into how elements are created in the universe.
The team built the device, called SOLARIS (Solenoid Spectrometer Apparatus for Reaction Studies), at the university’s Facility for Rare Isotope Beams (FRIB), a DOE Office of Science user facility. Experiments using SOLARIS are revealing information about the nuclear reactions that create the elements.
During a SOLARIS experiment, scientists direct a beam of particles at a target inside a chamber. When the particles collide with the target, reactions occur and neutrons or protons are either added or removed from the beam. By recording the energy and angle of the particles emitted from those collisions, SOLARIS enables scientists to gather information about the structure of the nuclei. SOLARIS is unique in that it can use both high- and low-intensity beams to measure at high accuracy. This dual functionality permits an even broader range of experiments, offering new insights into questions that have mystified nuclear physicists for a century.
U-M collaborates with Argonne on anti-leukemia compound
An anti-cancer compound developed by University of Michigan (U-M) researchers could lead to a pivotal treatment, according to U-M researchers Jolanta Grembecka and Tomasz Cierpicki, who were the senior authors of the study in the Journal of Clinical Investigation in December 2019. The research demonstrated strong efficacy in mouse models of acute leukemia that represented up to 40% of human acute myeloid leukemia patients. The U-M team, along with colleagues from several institutions, furthered their research by using the high-brightness X-rays from the Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility.
The compound, called MI-3454, inhibits the leukemia-related protein, called menin, which plays a critical role in a subset of acute leukemia with poor clinical outcomes. MI-3454 offered a complete remission of leukemia in mouse models derived from the leukemia patient samples with chromosomal translocations in the Mixed Lineage Leukemia (MLL1) gene. These genetic rearrangements are found in 5% to 10% of adult acute leukemia patients and in 80% of acute lymphoblastic leukemias in infants. The research showed the mice lived longer and showed no signs of leukemia months after the treatment stopped. The compound also may be used for prostate cancer and Ewing sarcoma, the study said.
Argonne‘s electric battery technology boosts Michigan auto industries
The nickel-manganese-cobalt (NMC) blended cathode — developed by DOE’s Argonne -- enabled the widespread rollout of lithium-ion batteries throughout the marketplace. Since the development of this material nearly 15 years ago, Michigan-based industries have been at the epicenter of building the batteries that use this material and the cars that use the batteries. Argonne’s NMC blended cathode has led to multiple commercialization agreements and the building of manufacturing plants in Michigan and Ohio. NMC has become a prominent cathode material in the transportation market, with applications ranging from power tools to hybrid electric vehicles and could enable large-scale energy grid storage.
Another battery innovation comes from Tom Guarr, co-founder of Jolt Energy Storage Technologies LLC and a researcher at the Michigan State University Bioeconomy Institute in Holland, Michigan, who was chosen to participate in Argonne’s Chain Reaction Innovations in April 2018. Guarr is developing a small molecule that enables the production of a novel flow cell battery for energy storage. His work could revolutionize the field of energy storage.